A new paper in Cell journal refines and functionally investigate the reproductive growth of the mouse intestine with modern genetic tools. Authors describe its cellular features and uncover underlying molecular changes and triggers. Martin Schwarzer and Umesh Kumar Gautam from Laboratory of Gnotobiology MBÚ were international collaborators in this study led by Irene Miguel-Aliaga at the Francis Crick Institute in UK.
Laboratory of Gnotobiology focuses on breeding of germ-free mice and gnotobiotic mice. Their experiments showed that the intestinal remodeling during pregnancy is not dependent on microbiota – the intestinal elongation in pregnancy and during lactation happens also in germ-free mice.
PUBLICATION
Growth of the maternal intestine during reproduction
Ameku et al., Cell (2025); DOI: 10.1016/j.cell.2025.02.015
HIGHLIGHTS
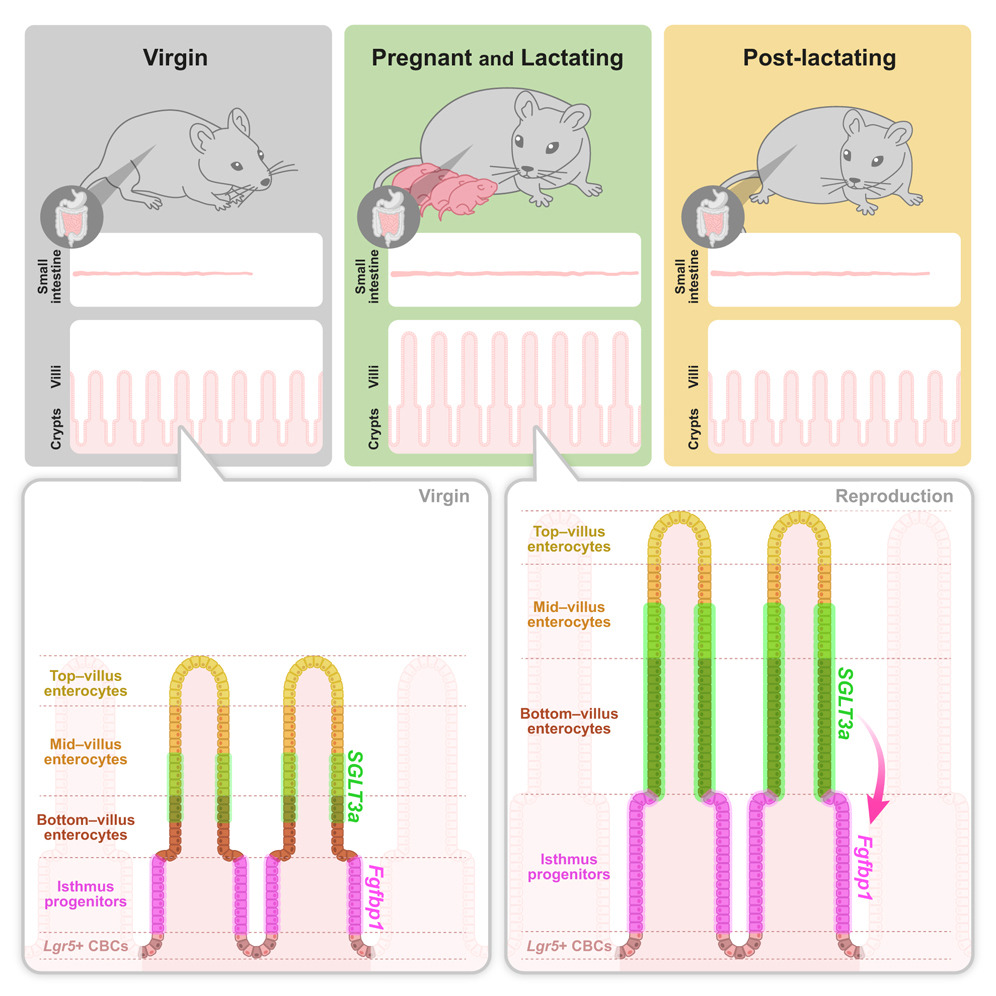
- Resizing of the maternal intestine in pregnancy has unique and anticipatory features
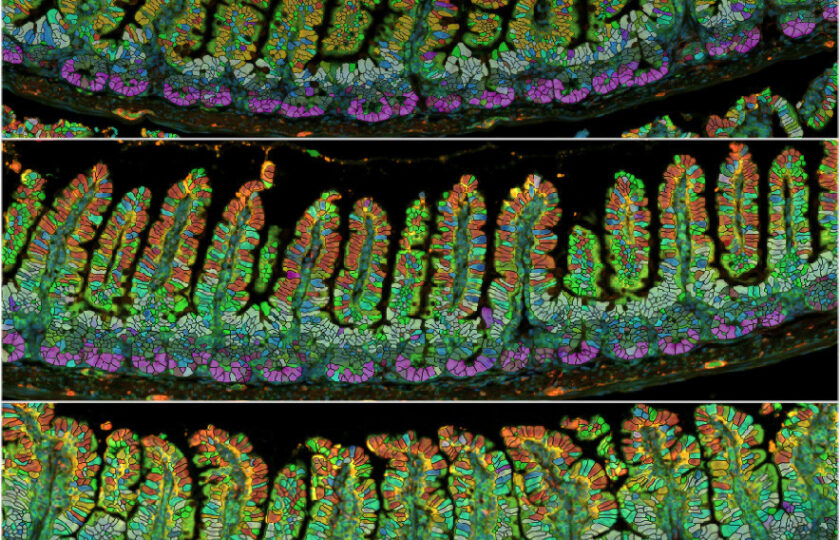
- Intestinal elongation is partially irreversible, and villus growth is fully reversible
- Sodium- and proton-sensitive SGLT3a transporter is induced by pregnancy in enterocytes
- SGLT3a extrinsically sustains Fgfbp1+ isthmus progenitor expansion and villus growth
SUMMARY
The organs of many female animals are remodeled by reproduction. Using the mouse intestine, a striking and tractable model of organ resizing, we find that reproductive remodeling is anticipatory and distinct from diet- or microbiota-induced resizing. Reproductive remodeling involves partially irreversible elongation of the small intestine and fully reversible growth of its epithelial villi, associated with an expansion of isthmus progenitors and accelerated enterocyte migration. We identify induction of the SGLT3a transporter in a subset of enterocytes as an early reproductive hallmark. Electrophysiological and genetic interrogations indicate that SGLT3a does not sustain digestive functions or enterocyte health; rather, it detects protons and sodium to extrinsically support the expansion of adjacent Fgfbp1-positive isthmus progenitors, promoting villus growth. Our findings reveal unanticipated specificity to physiological organ remodeling. We suggest that organ- and state-specific growth programs could be leveraged to improve pregnancy outcomes or prevent maladaptive consequences of such growth.
Graphical abstract