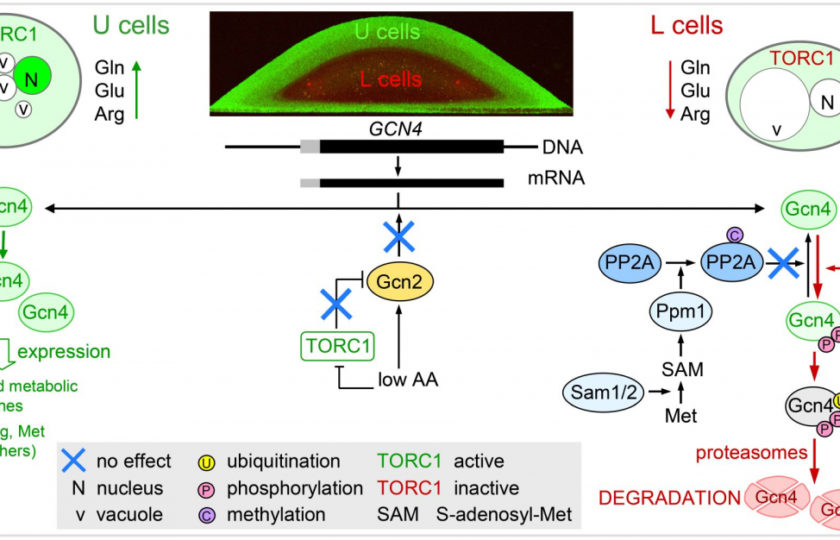
Gcn4p is a conserved transcription factor of the AP-1 family that is involved in the regulation of many cellular processes, including cell proliferation and aging, stress response and nutrient availability in yeast and mammals. In liquid cultures consisting of uniform yeast cells, Gcn4p activity increases with amino acid deficiency and is rapidly eliminated with amino acid excess. The main regulator of Gcn4p synthesis there is the protein kinase Gcn2p, which transmits the nutrient stress signal and thus initiates the translation of the GCN4 mRNA.
In this work, we have demonstrated Gcn4p activity specific to a spatially localized subpopulation of vital U cells in structured yeast colonies. Surprisingly, this transcription factor was not active in other cells of the colony, including a subpopulation of starving L cells. Using a combination of in situ microscopy and extensive analyses of modified strains, we found that this cell-specific activity of Gcn4p is determined by a post-translational regulation that depends on differential proteasomal degradation of nuclear Gcn4p in different cell types of differentiated colonies.
This regulation, which is independent of Gcn2p kinase or other transcriptional or translational regulation, results in stabilization and increased activity of Gcn4p in the presence of amino acids, whereas its degradation occurs in amino acid scarcity. This regulation resembles in some aspects the regulation of the mammalian ortholog of Gcn4p, the transcription factor ATF4, under the specific conditions of insulin regulation.
Reference: Váchová L, Plocek V, Maršíková J, Rešetárová S, Hatáková L, Palková Z. (2024) Differential stability of Gcn4p controls its cell-specific activity in differentiated yeast colonies. mBio 15: e0068924 doi: 10.1128/mbio.00689-24.