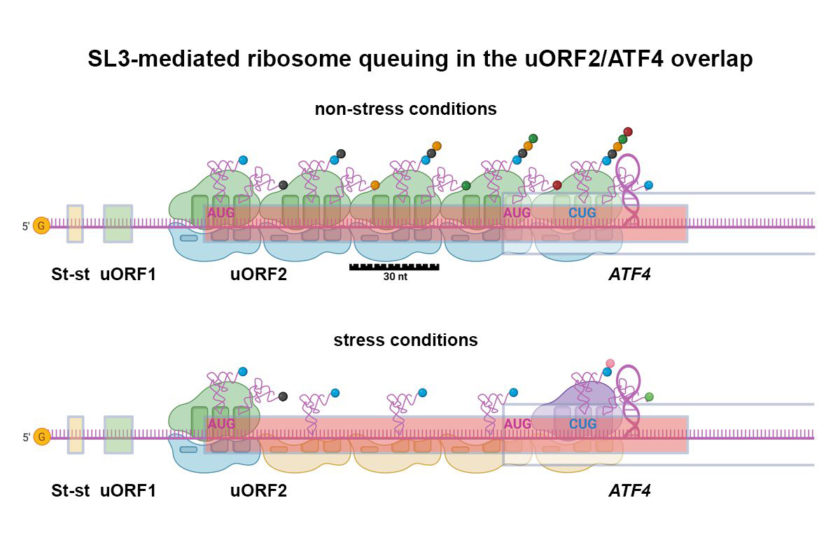
The cell, like the human body, is subjected to stress in adverse conditions. The ATF4 protein is an important tool for the cell to cope with stress. The Laboratory of Gene Expression at the Institute of Microbiology of the CAS has studied this “anti-stress” protein and the mechanism of its production for the past ten years. Scientists have described in detail the molecular mechanism by which the cell instantly turns on the ATF4 synthesis under various stressors.
“While during a stressful situation, the synthesis of the vast majority of proteins in the cell practically stops, the synthesis of ATF4, on the contrary, goes into full swing. This happens thanks to special regulatory elements that are located at the beginning of the mRNA that codes for this protein,” explains the head of the laboratory Leoš Shivaya Valášek from the Institute of Microbiology of the CAS.
A protein that controls the fate of stressed cells
Cells have developed different mechanisms how to respond to stressful situations. One of the examples are signaling pathways that, after activation, specifically influence and alter the behavior of the cell.
The ATF4 protein lies directly at the intersection of several of these signaling pathways and determines what happens to the stressed cell in several ways.
“This protein will allow the cell to completely reprogram its activity; in an instant to stop everything and focus all energy on coping with the given stress. If the cell fails to adapt within a certain time frame, ATF4 triggers so-called programmed cell death, so that the stressed cell does not become dangerous for its surroundings – e.g. malignant, i.e. dividing uncontrollably,” explains Anna Smirnová from the Institute of Microbiology of the CAS.
The newly described mechanism significantly extends the previous theory
The mechanism by which the cell synthesizes ATF4 in stressful situations was described in two prestigious publications as early as 2004 by two scientific groups – the group of Dr. Ronald Wek at the Indiana University School of Medicine and the group of Dr. David Ron at the University of Cambridge.
“For a long time, it was believed that this long-standing mystery had been solved once and for all. Over time, however, the results of other studies began to accumulate, which indicated that the molecular mechanism of translational control of this important fighter against stress is much more complex than it initially seemed,” says Valášek.
Based on these growing doubts, scientists at the Institute of Microbiology of the CAS launched their ten year long research project. “Serendipitously, the day after this paper was accepted, an invited lecture by Dr. David Ron, a fellow of the British Royal Society, was held at the Institute of Organic Chemistry and Biochemistry of the CAS. I met him there, handed him the newly accepted manuscript with a personal dedication, wishing him a smooth journey back home with this manuscript in hand,” adds Valášek.
Tailoring future therapies
This discovery is important when researching new medical therapies. “Given that deregulated ATF4 synthesis accompanies various pathological conditions, including cancer, our work clearly shows that when considering appropriate therapies that target ATF4, the complexity of controlling the synthesis of this key life-or-death regulator of stressed cells must be taken into account,” adds Valášek.
Kontakt:
dr. rer. nat. Leoš Shivaya Valášek, DSc.
Mikrobiologický ústav AV ČR